Abstract
Nanostructured chromium-based coatings have been researched. In some cases, it is expedient to use vacuum methods of deposition of chromium coatings by methods of thermal evaporation of pure chromium from tungsten coils or by ion-plasma (magnetron) sputtering method. Due to the low deposition temperature of coatings, there is a possibility of their formation on metallic and non-metallic materials. Based on the above, it is necessary to note the relevance of the study of the technology for the formation of chromium-based coatings by the ion-plasma method. To apply wear-resistant chromium coatings to samples made of R6M5 steel, the method of ion-plasma (magnetron) sputtering with a preliminary treatment of the surface with an ion source was used. The thickness, adhesive strength and corrosion resistance of chromium-based coatings were determined. Electron microscopy methods have been used to study the morphology of the surface and the size of nanoparticles in the structure of chrome coatings. It was revealed that depending on the modes of formation, the coating consists of nanoparticles with sizes from 15 nm to 230 nm.
1. Introduction
Recently, there has been an increased interest in the study of materials with a nanocrystalline structure due to a decrease in the size of crystals below a certain threshold value leads to a radical change in the physical and chemical properties of these materials. The strongest change in the properties of nanomaterials is achieved in the range of crystallite sizes up to 100 nm. The technology of producing thin films and coatings can be attributed to nanotechnology. Thin films and coatings can be produced by PVD and CAD. Thus, the well-known coatings of titanium carbide and nitride are obtained by ion-plasma deposition, which leads to the formation of nanocrystalline structure [1, 2].
Chromium and chromium nitride-based coatings are used in industry as a hard thin film to protect parts of cutting and processing tools, have excellent wear resistance, high hardness, sufficient strength, good adhesion to the substrate, high corrosion resistance and heat resistance up to 600 °C [3-6]. Chromium electroplating on steels and alloys, which are characterized by high chemical and mechanical resistance, is widely used in industry, but they are obtained using environmentally harmful chemical technology that requires treatment facilities. Electroplating uses toxic hexavalent chromium, and significant tensile stresses are generated, which lead to the appearance of a lattice of cracks in the coating immediately after deposition [7, 8].
Chromium nitride-based coatings are hard coatings that can be successfully applied as wear-resistant coatings to protect the wearing surfaces of parts and tools. In work [9], multilayer coatings based on chromium nitride and chromium carbon-nitride were studied. Multilayer alternating coating layers based on CrN/CrCN are formed by the PVD (CAD-cathode arc deposition) method. Experimentally established that the wear resistance of multilayer coatings based on chromium nitride and chromium carbonitride is higher than the individual CrN and CrCN coatings. A 0.1 μm thick chromium sublayer was used as a sublayer.
The novelty of this study lies in taking into account the ion cleaning of the surface of the samples before applying the chromium coating, as well as in varying the modes of the technological process of coating deposition using a modernized vacuum unit.
In this work, the composition, microstructure and residual stresses, as well as the properties of chromium coatings obtained by the PVD (physical vapor deposition) method were studied [10, 11]. CrN coatings are produced on a P6M5 steel substrate by the physical vapor deposition (PVD) method with extended capabilities. Based on the above, it is necessary to note the relevance of studying the technology of forming coatings based on chromium and chromium nitride by the ion-plasma method.
2. Methods
To apply wear-resistant chromium coatings to samples made of P6M5 steel, the method of ion-plasma (magnetron) sputtering with preliminary treatment of the surface with an ion source was used. The vacuum working chamber of the UV-75R-1 unit was equipped with a magnetron sputtering source of the MAG-5CM type and an ion source with a cold cathode creating a tubular ion flow.
The cathode of the magnetron sputtering source was a disk with a diameter of 130 mm, consisting of a copper base and a layer of chromium (VCU2) about 5 mm thick, which was subjected to sputtering. Argon ions were used to treat the surface of the products. Ion treatment was used to improve the adhesive strength of chrome coatings with the substrate. The relative position of the devices for processing products in the vacuum working chamber is shown in Fig. 1.
After reaching an initial vacuum level of approximately 10-2 Pa, the fixture with movable samples and the control plate were treated using an ion source in the following modes, as shown in Table 1.
Fig. 1Mutual arrangement of devices in a vacuum chamber: 1 – cold cathode ion source; 2 – magnetron sputtering device; 3 – rotating equipment; 4 – working chamber
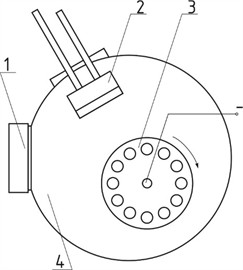
Table 1Ion surface treatment modes
Technological modes | Quantities and units of measurement |
Working gas pressure (argon), | 2·10-1 Pа |
Discharge voltage, | 4,5 kV |
Discharge current of the ion source, | 80-100 mA |
Current density on the treated surface, | up to 1 mА/sm2 |
Surface treatment time of the samples, | 3 minutes |
Once the ion source was triggered, the magnetron source was activated, and the setup was then rotated to the position opposite to the sputtering source. Ion-plasma chromium coating deposition was performed in the following technological modes (Table 2).
Table 2Vacuum chrome deposition modes
Technological modes | Quantities and units of measurement |
Distance from the cathode to the surface of the samples, b | 120-130 mm |
Working gas pressure (argon), P | 2·10-1 Pа |
Cathode voltage during sputtering, V | –550 V |
Discharge current during sputtering, I | 2,0-2,5 A |
Chromium coating deposition time, t | 5-20 minutes |
The temperature of the samples during coating deposition did not exceed 150 °C. The thickness of the vacuum-deposited chromium coatings was determined from a silicon control plate that was subjected to the same processing conditions as the steel samples.
Thickness measurement was carried out on a micro-interferometer of the MII-4 type according to a standard procedure. For this purpose, steps in the form of strips or islands of chrome were formed on a polished silicon plate with a deposited solid chrome coating. A chemically resistant varnish (FP-383) was applied to the surface of the coatings and chromium was etched in concentrated sulfuric acid in the presence of aluminium (an aluminium probe was lowered to the surface of the chromium section to activate etching). The varnish layers were then removed in dimethylformamide.
The interferometer was used to study the emerging step at the coating - silicon substrate boundary. Since both surfaces had a high reflectivity and a slight roughness in the boundary area, there was a clear interference pattern. The thickness of the coatings’ was determined by the displacement of the interference pattern lines according to the well-known Eq. (1):
where is the number of displacement lines of the interference pattern, is the wavelength of light (approximately 550 nm). An offset of 1 line corresponds to a step of about 0.27 μm, so it is convenient to identify steps of the order of 1-2 μm. According to the measurements, the maximum chromium coating thickness was about 2.4 μm for a deposition time of 20 minutes. Studies have shown that the thickness of chromium coatings is proportional to the deposition time in the interval of 2-20 minutes. For the first time, 1-2 minutes of chromium spraying, the coating deposition rate is below average.
The maximum thickness of the chromium-based coating was measured to be about 2.4 μm for a deposition time of 20 minutes. Studies have shown that the thickness of chromium coatings is proportional to the deposition time in the interval of 2-20 minutes.
The adhesive strength of the coatings was studied by the method of normal detachment of glued metal rods from the surface of the coatings. The coated specimen was placed in a cassette with vertical 7 rods (diameter of the glued part 1 mm), which were glued to the coating and torn off using a tensile machine. Studies have shown that the adhesive strength exceeded 70-80 MPa (700 kg/сm2), as the rupture occurred on the glue.
Corrosion problems seen in coating materials are usually the result of corrosive reagents penetrating through defects and entering the substrate. The application of a coating, especially a multi-layer coating, improves the corrosion resistance of the material. Cr and CrN-based coatings with a dense structure and finely dispersed crystals make them less permeable to corrosive environments. The absence of direct diffusion channels is due to the non-columnar structure, resulting in a significantly reduced rate of oxygen diffusion through the coatings.
Based on this, we studied the chemical resistance of samples with chromium-based coatings in a solution of nitric and hydrofluoric acid, which showed their high corrosion resistance. For coatings with a thickness of less than 1μm, the number of corrosion points (pores in the coating) was 5-10 times greater than for coatings with a thickness of 2.3-2.5 μm. As the experiment shows, with a change in the thickness of a nanostructured coating based on chromium, corrosion resistance changes several times, which implies the existence of through channels or their absence with an increase in the thickness of the coatings. Another important feature of nanostructured chrome coatings is the structural components, i.e. the size of the nanoparticles in the coatings, which have a significant impact on the corrosion resistance of the ‘coating-substrate’ system.
High-temperature oxidation resistance is one of the most attractive properties of nano-coatings. This property is highly dependent on the phase and chemical composition of the film. By interrupting the continuous path along the grain boundaries from the coating surface through the entire thickness to the substrate, the oxidation resistance can be increased. This can be achieved if the structure of the film is amorphous [12].
To determine the wear resistance of the coatings, comparative studies were conducted on the wear resistance of tools – taps with a chromium coating thickness of approximately 0.8-1.0 μm. Studies of several batches of products showed a 2-3 times increase in their wear resistance compared to standard tools. The tools were used for processing copper strips at the manufacturing plant of JSC “Elektroapparat-Elektroshchit”. The wear resistance of the taps was determined by the number of parts processed: the tool without coating processed 20 parts, while the coated tool processed 42-45 parts.
Considering all these features, electron microscopy was used to study the surface morphology and particle sizes in the structure of chrome coatings.
Information on the surface relief and size of agglomerates of chromium nanoparticles was obtained using transmission electron microscopy. Two-step Pt/C replicas were obtained for surface study [13, 14].
Electron microscopic studies have shown that structures with chromium nanoparticles are observed for samples No. 1 (Fig. 2) and No. 2 (Fig. 3). It is shown that during the formation of nanostructures from Cr with the maximum amount of chromium-containing phase, spherical chromium nanoparticles with a size of 66 nm to 130 nm are formed under 1-condition (Fig. 3), and for a sample obtained under 2-condition, larger chromium nanoparticles (66-200 nm) are observed (Fig. 4).
Fig. 2Electron microscopic images of chrome-plated specimens: Sample No. 1, 15000 H, 1 cm = 660 nm; P= 3·10-2 Pa
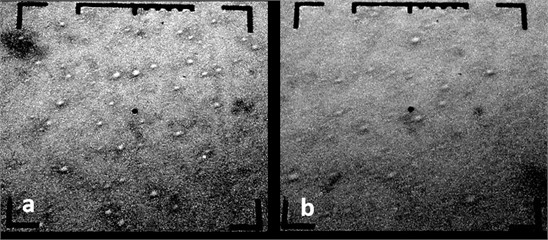
Fig. 3Electron microscopic images of chrome-plated specimens: Sample No. 2, 15000 H, 1 cm = 660 nm; P= 3·10-3Pa
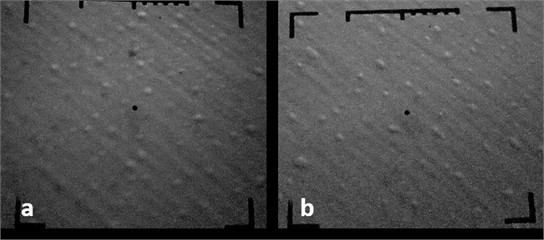
Fig. 4Electron microscopic images of chrome-plated specimens: Sample No. 2, 15000 H, 1 cm = 660 nm; P= 3∙10-3Pa
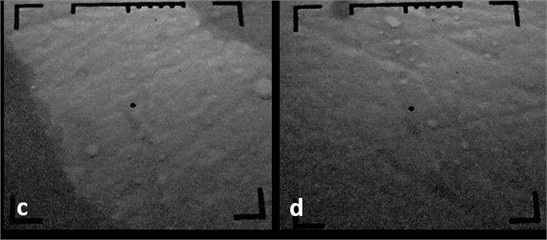
On the third sample, a nanostructure of inhomogeneous size is observed, nanoparticles with sizes from 15 nm to 230 nm. Moreover, it should be noted that the number of nanoparticles in this sample is much less than in the previous two samples. Based on the conducted research, it is possible to conclude about the wide possibilities of use of vacuum-precipitated magnetron sputtering of chromium coatings instead of electroplating coatings. In connection with the expansion of the fields of application of ultra-disperse materials, there is a need not only to transfer the substance to an ultra-disperse state, but also to create nanomaterials with a certain set of properties. The use of ultrafine metals and alloys is largely limited by the physical and chemical characteristics of these materials, namely: thermos-temporal instability, a wide range of particle sizes, and a significant content of oxides and impurities.
3. Results and discussions
Electron microscopic studies have shown that structures with chromium nanoparticles are observed for the samples (Fig. 2-4). It is shown that during the formation of nanostructures from Cr with the maximum amount of chromium-containing phase, spherical chromium nanoparticles with a size of 45 nm to 130 nm are formed under 1-condition (Fig. 5), and for a sample obtained under 2-condition, larger chromium nanoparticles (66-200 nm) are observed (Fig. 6).
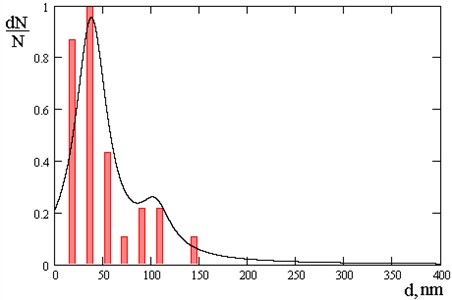
Fig. 5Number of nanoparticles: 97 % – 45 nm, largest quantity 50 nm and 100 nm of nanoparticles
4. Conclusions
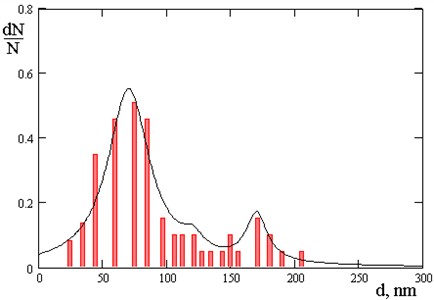
It has been revealed that by varying the technological modes of the process, it is possible to adjust the size and volume number of nanoparticles in chrome coatings, for example, at a pressure in a vacuum chamber 3∙10-2 Pa - the size of nanoparticles ranges from 50 to 100 nm, and at a pressure of 3∙10-3 Pa - the volume content of nanoparticles depending on the size is distributed as follows: nanoparticles with dimensions of 70 nm in coatings are present in the amount of 55 %, 120 nm – 15 %, and 170 nm – 30 %.
Fig. 6Number of nanoparticles: 55 % 70 nm; 15 % 120 nm; 30 % 170 nm
It has been established that with an increase in thickness, the corrosion resistance of chrome coatings changes several times. With an increase in the thickness of coatings from 1 μm to 2.3-2.5 μm, the corrosion resistance increases by 5-10 times.
Studies have shown that the thickness of chromium coatings is proportional to the deposition time in the interval of 2-20 minutes.
In connection with the expansion of the fields of application of ultra-disperse materials, there is a need not only to transfer the substance to an ultra-disperse state, but also to create nanomaterials with a certain set of properties.
Based on the conducted research, it is possible to draw a conclusion about the wide possibilities of use of vacuum-precipitated magnetron sputtering of chromium instead of electroplating.
References
-
A. I. Gusev and A. A. Rempel, Nanocrystal Materials. (in Russian), Moscow: Fizmatlit, 2001.
-
I. P. Suzdalev, Nanotechnology: Physical-Chemistry of Nanoclusters, Nanostructures and Nanomaterials. (in Russian), Moscow: KomKniga, 2006.
-
C.-M. Suh, B.-W. Hwang, and R.-I. Murakami, “Behaviors of residual stress and high-temperature fatigue life in ceramic coatings produced by PVD,” Materials Science and Engineering: A, Vol. 343, No. 1-2, pp. 1–7, Feb. 2003, https://doi.org/10.1016/s0921-5093(02)00327-1
-
F. R. Lamastra, F. Leonardi, R. Montanari, F. Casadei, T. Valente, and G. Gusmano, “X-ray residual stress analysis on CrN/Cr/CrN multilayer PVD coatings deposited on different steel substrates,” Surface and Coatings Technology, Vol. 200, No. 22-23, pp. 6172–6175, Jun. 2006, https://doi.org/10.1016/j.surfcoat.2005.11.013
-
R. K. Saydakhmedov, K. K. Kadyrbekova, and A. I. Kamardin, Nanostructured Coatings and Modern Methods of Material Processing. (in Russian), Tashkent: Fan, 2012.
-
R. K. Saydakhmedov and K. K. Kadyrbekova, “The properties of protective nanocoatings based on chromium,” (in Russian), Metallurgy of Machine Building, No. 5, pp. 29–30, 2011.
-
K. K. Kadirbekova, “Development of effective compositions and optimal technological modes, obtaining composite nanostructured coatings by a physical method,” Tashkent, 2018.
-
F. G. Arieta and D. T. Gawne, “The wettability and durability of chromium plating,” Surface and Coatings Technology, Vol. 73, No. 1-2, pp. 105–110, Jul. 1995, https://doi.org/10.1016/0257-8972(94)02371-9
-
A. Gilewicz, B. Warcholinski, P. Myslinski, and W. Szymanski, “Anti-wear multilayer coatings based on chromium nitride for wood machining tools,” Wear, Vol. 270, No. 1-2, pp. 32–38, Dec. 2010, https://doi.org/10.1016/j.wear.2010.09.002
-
P. M. Perillo, “Properties of CrN coating prepared by physical vapour deposition,” American Journal of Materials Science and Application, Vol. 3, No. 2, pp. 38–43, 2015.
-
T. H. Lippitz, “XPS investigations of chromiumnitride thin films,” Surface and Coatings Technology, Vol. 200, pp. 250–253, 2005.
-
N. Abdujabarov, R. Shokirov, J. Takhirov, and S. Bobomurodov, “Mechanical properties of V95P alloy wire after high-temperature annealing,” in Asia-Pacific Conference on Applied Mathematics and Statistics, Vol. 2471, p. 030004, Jan. 2022, https://doi.org/10.1063/5.0090031
-
A. Sverdlin and N. Abdujabarov, “Heat treating aluminum for rivets and bolts,” Heat Treating Progress, Vol. 5, No. 4, pp. 48–50, 2005.
-
N. Azarenkov, V. Beresnev, D. Pogrebnyak, L. V. Malikov, and P. V. Turbine, Nanomaterials, Nanocoatings, Nanotechnology. (in Russian), Kharkov: KhNU names V.N. Karazina, 2009.
About this article
The authors have not disclosed any funding.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no conflict of interest.
