Abstract
When producing steel, the enterprise faces difficulties in removing sulfur from the melt. In turn, an increased sulfur content in the finished product leads to a decrease in the values of some mechanical properties. This work analyzes the desulfurization process of 20GL grade steel in an induction crucible furnace. The influence of sulfur on the impact toughness of steel and the factors influencing sulfur removal are considered. Calculated data and industrial melting data were obtained, the difference between these data was explained, and recommendations were made based on this data. The parameters of steel smelting technology, which determine the chemical composition and structure, are the main factors contributing to high mechanical properties. The relevance of the work lies in the need to improve and develop the desulfurization process, which allows for the production of steel with low sulfur content and high mechanical properties.
1. Introduction
The main activity of “Foundry and Mechanical Plant” JSC is the production of parts for railway transport. The main parts of these are the side frame and the overhead beam, which are cast from 20GL steel. To improve the quality of 20GL steel, work was carried out using alloying. However, doping increases strength with a decrease in plasticity. This steel must have sufficient plasticity and impact toughness. Also, steel smelting includes processes of refining from harmful impurities such as oxygen, sulfur, phosphorus.
The side frame is considered the load-bearing element of the wagon bogies. The side frame withstands static and dynamic vertical loads (from the weight of the wagon and cargo, from impacts when passing unevenness of the track) and longitudinal loads (traction forces during uniform movement of the train, forces during collision of wagons), as well as is subjected to the action of the torque when passing wagons into curves. At the same time, the main part of the dynamic vertical loads is cyclical, and the fatigue strength of the side frames (the ability to resist the effects of cyclical loads for a long time) is the main characteristic of their operational reliability [1].
2. Experimental analysis
The side frame (Fig. 1(a)) and the overhead beam (Fig. 1(b)) are the main parts of railway transport.
The chemical composition and mechanical properties of 20GL steel according to GOST 32400-2013 are presented in Tables 1 and 2.
The negative influence of sulfur on the properties of steel is determined by its physicochemical interaction with iron, i.e., high solubility in liquid melt and very low solubility in solid alpha-iron. The melting point of the eutectic Fe-FeS is 975 °C. During crystallization in the interdrital spaces, the melt is enriched with sulfur. Also, sulfur affects the properties of the metal through sulfide inclusions, which are formed as a consequence of metallurgical processes of binding sulfur into compounds stronger than FeS [2].
Table 1Chemical composition of 20GL steel, (% by mass.)
C | Si | Mn | Al | P | S | Cr | Ni | Cu | Fe |
No more than | |||||||||
0,17-0,25 (–0,020) | 0,30-0,50 (±0,10) | 1,10-1,40 (±0,10) | 0,020-0,060 (+0,005) | 0,020 (±0,005) | 0,020 (±0,005) | 0,30 (+0,20) | 0,30 (+0,30) | 0,60 | rest |
Table 2Mechanical properties of 20GL steel
Fluidity limit, MPa | Temporary resistance, MPa | Relative extension, % | Relative narrowing, % | Impact toughness, KCV-60, kJ/m2 |
No less than | ||||
343 | 510 | 18 | 30 | 200 |
Fig. 1a) Side frame and b) overhead beam

a)

b)
When steel is cooled, sulfides are formed due to a decrease in the solubility of sulfur. Basically, iron sulfides (FeS) and manganese sulfides (MnS) are formed, and sulfur also forms sulfides of other elements. Iron sulfide has a melting point of 1188 °C, however, an easily melting eutectic with a melting point of 988 °C forms in the metal. At high temperatures, the eutectic melts, resulting in the steel's brittleness. Even a relatively low sulfur content in the metal can lead to a decrease in mechanical and technological properties due to a tendency towards liquation [3].
During crystallization and cooling of steel, sulfur is released from the solution as inclusions of sulfides or oxysulfides of FeS∙FeO due to reduced solubility. Since the release of these inclusions occurs at the end of hardening, they are distributed along the boundaries of the grains of the already formed crystals, weakening their bonding, due to which the properties of the metal deteriorate. At room temperature and close to it, sulfide inclusions reduce the mechanical properties of steel-plasticity, impact toughness [2].
Fig. 2 shows the influence of sulfur and phosphorus on the impact toughness of steel.
As can be seen from the experimental data, as the sulfur content in the steel increases, the impact toughness decreases significantly. Therefore, steel with high impact toughness can be to obtain by reducing the sulfur content to low levels.
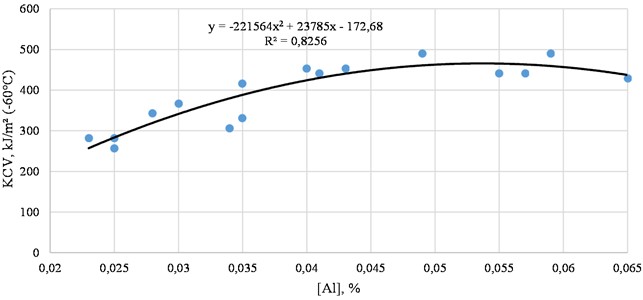
As can be seen from Fig. 3, the impact toughness level increases with increasing aluminum content. This can be explained by the fact that aluminum interacts with oxygen and nitrogen, reducing their content in the solid solution. As nitrogen and oxygen are in solid solution, their solubility decreases upon cooling, forming brittle phases in the form of oxides and nitrides along the grain boundaries, inhibiting grain growth. The separation of excess aluminum along the grain boundaries with the formation of non-metallic inclusions affects the mechanical properties. The resulting segregation causes intergranular brittleness, which leads to a decrease in impact toughness [4]. In steels deoxidized with aluminum, sulfide inclusions, separating along the boundaries of the primary crystallites, reduce their cleavage and thereby ensure a decrease in the plasticity and viscosity of the metal. Therefore, the higher the sulfur concentration in the metal, the more it is contaminated with non-metallic inclusions and the lower the plasticity indicators [5].
Fig. 2Dependence of the total harmful impurity content on the impact toughness of steel (actual data)
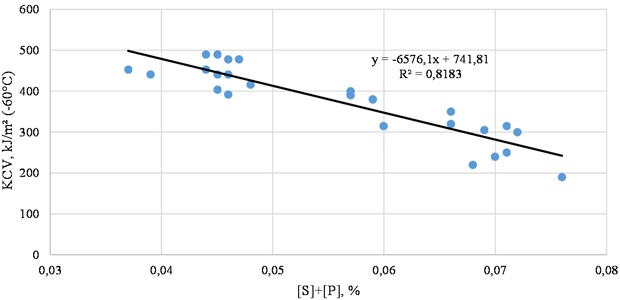
Fig. 3Dependence of aluminum on the impact toughness of steel (actual data)
By the nature of reduction and chemical composition, three types of sulfides can be formed in steel.
Type 1. Small globular manganese sulfides and oxysulfide inclusions. Such inclusions are formed in steels containing a small amount of aluminum.
Type 2. Eutectic manganese sulfides in the form of a continuous film. Such inclusions are arranged in the form of films, which leads to a decrease in the forces of intergranular bonds and a noticeable decrease in viscous and plastic characteristics.
Type 3. Large faceted manganese sulfides. They are formed with an increased content of aluminum.
Among them, type 2 sulfides have the most negative impact on steel properties. The aluminum content, which determines the formation of sulfides of one or another type, depends on the composition of the steel, as well as significantly on the carbon content [6].
The contribution of non-metallic inclusions formed when aluminum is added in an amount of 0.02-0.06 % to low impact toughness is small.
The main factors influencing the desulfurization process are the slag composition and quantity, the degree of steel deoxidation, the intensity of slag and metal mixing, the metal composition, and the bucket lining. Optimization of these technological factors ensures the effectiveness of the process [7].
Slag performs a number of technological functions, such as the assimilation of non-metallic inclusions, protection from secondary oxidation, and desulfurization. To perform such functions, the slag must have the following characteristics: basicity (CaO/SiO2) ˃ 5; content (SiO2) ˂ 10 % (mass.); content (FeO) + (MnO) ˂ 1.0 % (mass.); fluidity. To achieve such characteristics, it is necessary to use slag-forming materials (lime, fluorspar, alumina-containing materials) and reducing agents in different proportions [8].
Currently, the most modern desulfurizer is fluidized lime, calcium carbide, and granulated magnesium. Treating metal with fluidized lime has become widespread and is a good desulfurizer, allowing for the production of steel with a low sulfur content (up to 0.003 %). Fluidized lime has a fractionally dispersed composition, a high CaO content (over 95 %), and no other impurities [9].
Alkali-earth metals have high refining properties in relation to sulfur and oxygen dissolved in steel. The addition of alkali-earth metals to the melt not only ensures the reduction of sulfur and oxygen, but also contributes to controlling the nature and form of non-metallic inclusions, which allows obtaining a metal with optimal physical-mechanical and technological properties [5].
However, obtaining steel with low oxygen concentration is the main task, but not the only one. When oxidized with aluminum, cloud-like accumulations of sharp-angled particles form in the steels, leading to the formation of microcracks. Adding stronger reducing agents such as magnesium, calcium, and rare earth metals to steel leads to a significant decrease in oxygen, changes in the morphology of inclusions, and a reduction in the number of surface and internal defects in steels [10].
The oxygen content and the type of reducing agent influence the formation of inclusions in the steel. At the same time, it is necessary to prevent oxygen from entering the atmosphere. Therefore, blowing with an inert gas (argon) is very important. In addition, blowing allows for increasing the degree of desulfurization, averaging the chemical composition and temperature, and removing non-metallic inclusions. However, the gas intensity should not expose the metal, leading to its secondary oxidation [11].
Table 3 shows the average chemical composition of steel during production (actual data).
Table 3Chemical composition of steel at the furnace release (mass. %)
C | Si | Mn | Al | S | P | Fe |
0,214 | 0,356 | 1,157 | 0,042 | 0,020 | 0,023 | rest |
Deoxidation by aluminum is described by Eq. (1) [12]:
The equilibrium constant of the reaction is described by the Eq. (2):
where: – aluminum content in metal, %; – oxygen content in metal %; – aluminum oxide content in slag, %; – equilibrium constant of the reaction; – activity in slag; – aluminum activity in metal; – oxygen activity in metal; – aluminum activity coefficient in metal; – oxygen activity coefficient in metal; – metal temperature, K.
The degree of desulfurization is calculated using Eq. (3):
The figure shows that as the basicity of the slag increases, the sulfur content decreases. Also, the multiplicity of the slag significantly affects the removal of sulfur. For example, if the scale multiplicity is equal to one and basicity interval is from 1 to 5, the sulfur concentration varies from 0,037 % to 0,008 %.
The use of calcium-containing materials has become widespread. Calcium is a good deoxidizer and desulfurizer, and it also affects the type, shape, and distribution of non-metallic inclusions. The desulfurization process is significantly influenced by slags with a composition of CaO - 50-70 %, SiO2 - 5-10 %, Al2O3 - 20-30 %, MgO - 4-8 %.
To reduce the viscosity of the reducing slag, fluorspar is added to the metal. The use of fluorspar allows for the liquefaction of slag without reducing its basicity, which contributes to the removal of sulfur [14].
The addition of fluorspar provides deep desulfurization due to increased slag mobility. To direct slag, a solid slag mixture (SSM) of lime and fluorspar is used in a 3:1 or 4:1 ratio [7].
The final sulfur content depends on its content in the slag. With a decrease in sulfur content in the slag due to slag renewal, the concentration of sulfur in the melt decreases [7].
Consequently, an indispensable condition for obtaining steel with a particularly low sulfur content is not only the formation of a high-basic slag with a low FeO content and high fluidity, but also the deep oxidation of the metal [7].
Fig. 4 shows the dependence of the desulfurization degree on the residual aluminum content.
Fig. 4Dependence of the desulfurization degree on the residual aluminum content (calculated data)
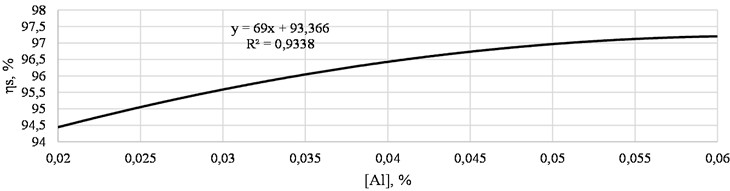
From the figure, it can be seen that during the decomposition of aluminum, the activity of oxygen in the steel decreases, since aluminum provides deep desulfurization and degree of desulfurization reaches 97,5 %.
The Fig. 5 shows dependence of the desulfurization degree on the residual aluminum content.
Fig. 5Dependence of the desulfurization degree on the residual aluminum content (actual data)
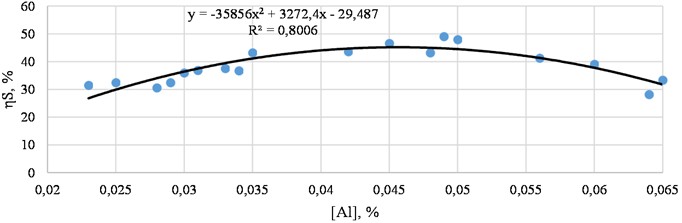
From Fig. 5, it can be seen that with an increase in the aluminum content, the oxygen content in the steel decreases and the degree of desulfurization increases. When oxidized with aluminum in the range of 0.035-0.055 %, aluminum significantly affects the degree of desulfurization and constitutes about 45 %.
When aluminum is added within the upper limits specified in GOST, the desulfurization process deteriorates, which is due to the increase in Al2O3 in the slag as CaO decreases [7].
Such a difference between the calculated and experimental results in the degree of desulfurization may be due to the high oxidation of the system, i.e., the low oxidation of the metal, which requires more decomposers or stronger decomposers. It can also be related to the intensity of mixing, insufficient slag discharge duration, and the inoptimal composition of the reducing slag.
Actual data were obtained from industrial smelting. From these data, it can be seen that in some melts, sulfur decreases to 0,017 %. During the desulfurization process, the sulfur content decreases from 0,037 % to 0,020 %.
To carry out the desulfurization process, the oxidizing slag was completely removed from the induction crucible furnace in the amount of 180-200 kg and a metal sample was taken for express analysis. Then, a 2000 kW furnace was switched on, and the metal was decomposed with silicon, manganese, and aluminum. Three times in equal portions, solid slag mixture (SSM) based on lime - 80-100 kg and fluorspar - 15-20 kg was administered, stirring the liquid bath with a metal rod for 25 minutes, then a second sample was taken for analysis [15].
The temperature at which the metal is released from the furnace into the ladle is 1650 °C. Upon reaching this temperature, the furnace switches to a thermostatic mode, holding the specified temperature for 15 minutes. The metal sample was taken after 15 minutes.
Average change in sulfur content during metal desulfurization is presented in Fig. 6.
Fig. 6Average change in sulfur content during metal desulfurization (actual data)
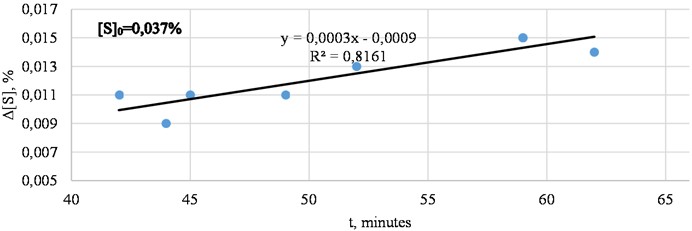
The longer the process duration, the better the sulfur is removed. During 45 minutes, 0,010 % of sulfur is removed. With increasing processing time, the desulfurization process improves, and after 60 minutes, sulfur removal is 0,015 %.
The decomposition process, removal of decomposition products, is carried out significantly faster due to the intensive movement of the melt in induction furnaces, which requires little time. It is also possible to conduct desulfurization in an induction furnace when unloading and supplying the reducing slag several times, increasing the fluorspar in the slag-forming mixtures. This leads to a decrease in lining stability and an increase in melting time [14].
In addition, during the reduction period, the high temperature of the metal and slag, and low oxidation, create favorable conditions for the saturation of the metal with nitrogen. Also, the lining of the furnace is severely worn out during prolonged storage. Therefore, it is necessary to carry out the recovery period in the shortest possible time. The composition of the metal for oxygen and sulfur allows for a reduction in the time required for technological operations such as oxidation, desulfurization, and alloying [16].
3. Conclusions
Desulfurization calculations were carried out, and experimental data on 20GL steel alloys were obtained, and the difference between the calculated and experimental data on the degree of desulfurization was analyzed. Calculations show that the degree of desulfurization reaches up to 97,5 %. However, in practice, 45 % of the sulfur is removed. The degree of desulfurization is influenced by the oxidation state of the system, as the desulfurization degree is approximately 45 % when oxidized with aluminum within the range of 0,035-0,055 %. It has been experimentally shown that the longer the desulfurization time, the higher the degree of sulfur removal. During 45 minutes, 0,010 % of sulfur is removed. Prolonged processing of steel allows for improved desulfurization process, and after 60 minutes, sulfur removal is 0,015 %. Calculations have also shown that the multiplicity of the slag significantly affects the removal of sulfur. For example, if the scale multiplicity is equal to one and the basicity interval is from 1 to 5, the sulfur content varies from 0,037 % to 0,008 %. In practice, when steel is processed with solid slag mixtures, the sulfur content decreases from 0,037 % to 0,020 %.
Based on this, a conclusion was made that the desulfurization process may be associated with insufficient oxidation by strong oxidizing agents, a smaller amount of slag-forming mixtures, a small amount of slag, insufficient slag discharge duration, and a low intensity of metal and slag mixing. In this regard, it is recommended to use strong reducing agents, complex reduction, increasing the amount of slag and the duration of slag discharge, forming the optimal slag composition, using more solid slag mixtures, treating the metal with alkaline earth metal alloys, as well as various methods of metal mixing.
References
-
T. B. Brylova, “Evaluation of the influence of liquation on crack formation of side frames made of 20GL steel: Technological support for repair and improvement of dynamic qualities of railway rolling stock,” in Proceedings of the Third All-Russian Scientific and Technical Conference with International Participation in Three Parts. Part 3, 2015.
-
M. Turakulov, N. Tursunov, and S. Yunusov, “Steeling of synthetic cast iron in induction crucible furnace taking into account consumption rate of carburizers,” in E3S Web of Conferences, Vol. 401, p. 05012, Jul. 2023, https://doi.org/10.1051/e3sconf/202340105012
-
T. Tursunov, N. Tursunov, and T. Urazbayev, “Investigation of heat exchange processes in the lining of induction furnaces,” in E3S Web of Conferences, Vol. 401, p. 05029, Jul. 2023, https://doi.org/10.1051/e3sconf/202340105029
-
O. Toirov and N. Tursunov, “Efficiency of using heat-insulating mixtures to reduce defects of critical parts,” in E3S Web of Conferences, Vol. 401, p. 05018, Jul. 2023, https://doi.org/10.1051/e3sconf/202340105018
-
D. Valieva, S. Yunusov, and N. Tursunov, “Study of the operational properties of the bolster of a freight car bogie,” in E3S Web of Conferences, Vol. 401, p. 05017, Jul. 2023, https://doi.org/10.1051/e3sconf/202340105017
-
L. Kuchkorov, N. Tursunov, and A. Avdeeva, “Improving physical and mechanical properties of bentonite clay from Navbahar clay deposit,” in E3S Web of Conferences, Vol. 401, p. 05020, Jul. 2023, https://doi.org/10.1051/e3sconf/202340105020
-
U. Rakhimov and N. Tursunov, “Development of technology for high-strength cast iron for manufacturing D49 head of cylinder,” in E3S Web of Conferences, Vol. 401, p. 05013, Jul. 2023, https://doi.org/10.1051/e3sconf/202340105013
-
T. Urazbayev, N. Tursunov, and T. Tursunov, “Steel modification modes for improving the cast parts quality of the rolling stock couplers,” in Problems in the Textile and Light Industry in the Context of Integration of Science and Industry and Ways to Solve Them: PTLICISIWS-2, Vol. 3045, p. 060015, Jan. 2024, https://doi.org/10.1063/5.0197361
-
U. T. Rakhimov, N. K. Tursunov, and S. E. Tursunov, “Improvement of production technology for spheroidal graphite cast iron with increased strength,” in Problems in the Textile and Light Industry in the Context of Integration of Science and Industry and Ways to Solve Them: PTLICISIWS-2, Vol. 3045, p. 060024, Jan. 2024, https://doi.org/10.1063/5.0197475
-
S. R. Seydametov, N. K. Tursunov, and S. P. Alimukhamedov, “Development of out-of-furnace steel treatment technology for the manufacture of railroad transport parts,” in Problems in the Textile and Light Industry in the Context of Integration of Science and Industry and Ways to Solve Them: PTLICISIWS-2, Vol. 3045, p. 060022, Jan. 2024, https://doi.org/10.1063/5.0197429
-
U. Ziyamukhamedova et al., “Investigating friction and antiwear characteristics of organic and synthetic oils using h-BN nanoparticle additives: a tribological study,” Lubricants, Vol. 12, No. 1, p. 27, Jan. 2024, https://doi.org/10.3390/lubricants12010027
-
C. Shekhar, M. F. Wani, R. Sehgal, U. Ziyamukhamedova, and N. Tursunov, “A novel ceramic reinforced metal matrix composite (Cu-Ni/TiC-CaF2): fabrication, microstructure, mechanical and tribological characterization,” Metallurgical and Materials Transactions B, Vol. 56, No. 2, pp. 1289–1315, Jan. 2025, https://doi.org/10.1007/s11663-024-03418-2
-
N. Tursunov, S. S. Saleem, U. Ziyamukhamedova, M. F. Wani, M. N. Bhat, and T. A. Mufti, “Microstructure, mechanical and tribological characterization of magnetron sputtering ZrN and ZrAlN coatings,” Tribology International, Vol. 202, p. 110295, Feb. 2025, https://doi.org/10.1016/j.triboint.2024.110295
-
C. Shekhar, M. F. Wani, R. Sehgal, S. S. Saleem, U. Ziyamukhamedova, and N. Tursunov, “Recent progress in particulate reinforced copper‐based composites: fabrication, microstructure, mechanical, and tribological properties-a review,” Advanced Engineering Materials, Vol. 27, No. 2, p. 2401748, Dec. 2024, https://doi.org/10.1002/adem.202401748
-
O. Toirov, N. Tursunov, S. Alimukhamedov, and L. Kuchkorov, “Improvement of the out-of-furnace steel treatment technology for improving its mechanical properties,” in E3S Web of Conferences, Vol. 365, p. 05002, Jan. 2023, https://doi.org/10.1051/e3sconf/202336505002
-
L. Kuchkorov, S. Alimukhamedov, N. Tursunov, and O. Toirov, “Effect of different additives on the physical and mechanical properties of liquid-glass core mixtures,” in E3S Web of Conferences, Vol. 365, p. 05009, Jan. 2023, https://doi.org/10.1051/e3sconf/202336505009
-
M. Turakulov, N. Tursunov, and S. Alimukhamedov, “Development of technology for manufacturing molding and core mixtures for obtaining synthetic cast iron,” in E3S Web of Conferences, Vol. 365, p. 05006, Jan. 2023, https://doi.org/10.1051/e3sconf/202336505006
-
O. Toirov and N. Tursunov, “Development of production technology of rolling stock cast parts,” in E3S Web of Conferences, Vol. 264, p. 05013, Jun. 2021, https://doi.org/10.1051/e3sconf/202126405013
-
A. Riskulov, K. Nurmetov, and J. Avliyokulov, “Material selection for vehicle brake chamber case with using computer methods of analysis,” in E3S Web of Conferences, Vol. 401, p. 02061, Jul. 2023, https://doi.org/10.1051/e3sconf/202340102061
-
S. Azimov, O. Toirov, B. Xalmurzayev, S. Tursunov, and K. Khujakhmedova, “Using a cooling hole to improve the performance of transport brakes,” in Problems in the Textile and Light Industry in the Context of Integration of Science and Industry and Ways to Solve Them: PTLICISIWS-2, Vol. 3045, p. 060016, Jan. 2024, https://doi.org/10.1063/5.0197365
-
M. Turakulov, N. Tursunov, and S. Yunusov, “New concept of cast iron melting technology in induction crucible furnace,” in E3S Web of Conferences, Vol. 401, p. 01060, Jul. 2023, https://doi.org/10.1051/e3sconf/202340101060
About this article
The authors have not disclosed any funding.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors declare that they have no conflict of interest.
