Abstract
A three-dimensional stochastic aggregate model of concrete was established using the Monte Carlo method, and a numerical simulation of chloride ion diffusion at the microscopic level was conducted. The study investigated the migration behaviour of chloride ions in concrete regarding mixing proportions and temperature. The results showed that compared to the simulation results at an ambient temperature of 20 ℃, the chloride ion diffusion coefficient increased by 31 % and 70.5 % for concrete at 25 ℃ and 30 ℃ at 28 days, respectively. The chloride ion penetration depth increased by 17.3 % and 34.9 % for concrete at 25 ℃ and 30 ℃, respectively. With a slag content of 10.4 %, 20.8 %, and 27.1 %, the chloride ion diffusion coefficient at 28 days decreased by 1.4 %, 2.7 %, and 4.1 %, respectively. With a fly ash content of 8.3 %, 16.7 %, and 25 %, the chloride ion diffusion coefficient at 28 days decreased by 2.1 %, 5.4 %, and 9.2 %, respectively. Both slag and fly ash can reduce the chloride ion diffusion coefficient in concrete, with fly ash showing better effectiveness than slag. A water-to-binder ratio of 0.4, combined with 27.1 % slag and 25 % fly ash as cement replacements, can effectively improve the resistance of concrete to chloride ion attack. The micro-scale finite element model of concrete, developed through Monte Carlo simulation, offers enhanced visualization of chloride ion penetration processes under varying mix proportions and temperature conditions.
Highlights
- The Monte Carlo method was employed to construct a 3D stochastic aggregate model of concrete, enabling mesoscale simulation of chloride ion erosion dynamics.
- The transport law of chloride ion under different temperature conditions was simulated.
- Both slag and fly ash reduce the chloride ion diffusion coefficient and enhance resistance, with fly ash demonstrating superior performance to slag.
1. Introduction
The durability of concrete structures is influenced by the penetration of chloride ions under marine environmental conditions, which leads to reinforcement corrosion through the concrete cover [1-4]. OHA [5] proposed a mathematical model based on a multiscale approach to describe the diffusion coefficient of chloride ions in different pore sizes, analyzing the influence of internal pore structure, including pore size distribution, saturation, chloride ion concentration, on the transport of chloride ions in concrete. Bary [6] considered the diffusion of chloride ions, calcium ions, sodium ions, potassium ions, sulfate ions and hydroxide ions, and derived a set of coupled mass balance equations to establish a simplified coupled model for simulating chloride ion erosion in cement paste and mortar. Cherif [7] developed a physicochemical model for the multi-component transport of (Cl–, Na+, K+, Ca2+, SO42–, Al(OH))4–, OH–) in cementitious materials, considering thermodynamic equilibrium, diffusion, and migration, through simulating actual seawater exposure conditions. The model was applied in case studies of steady-state chloride ion migration tests and NT Build 492 testing on cementitious pastes based on slag and/or Portland cement.
Concrete at a mesoscale level consists of aggregates, cement mortar, and an interface transition zone (ITZ). These three phases have their own characteristics in terms of chloride ion diffusion [8]. The ITZ is the transitional region between the cement mortar and aggregates, containing more cracks and voids. Therefore, the transport rate of chloride ions in the ITZ is much higher than that in the cement mortar [9, 10]. Du [11] established a 2D mesoscale model and found that aggregate distribution and shape have little influence on chloride ion diffusion in concrete. The diffusion properties of the ITZ and aggregate content significantly affect the diffusion of chloride ions in concrete, while the water-to-cement ratio has a notable impact. Currently, the migration behavior of chloride ions in concrete at a mesoscale level is mainly studied by considering concrete as a three-phase composite material composed of aggregates (AGG), cement mortar (CP), and the interface transition zone (ITZ), and constructing a 2D mesoscale model for chloride ion diffusion in concrete. These three phases have unique chloride ion diffusion characteristics, with different diffusion coefficients for chloride ions in each phase. Aggregates are relatively dense, and chloride ions barely penetrate them. Chloride ions can freely diffuse through the cement mortar and ITZ, with the ITZ having more voids and microcracks than the cement mortar, resulting in a significantly higher diffusion coefficient for chloride ions in the ITZ. Li [12] calculated the diffusion of chloride ions in cementitious materials with simultaneous chloride ion incorporation under freeze-thaw conditions and validated the mesoscale model using experimental data from coupled tests of chloride ion erosion and freeze-thaw cycles. The calculated chloride ion concentration distribution showed good agreement with the measured data compared to the typical results obtained by Fick’s law.
The diffusion behavior of chloride ions in concrete is influenced by environmental temperature, which affects concrete’s micro-pore structure and diffusion activation energy [13, 14]. With increasing temperature, the diffusion rate of chloride ions in concrete significantly increases [15, 16]. From the above analysis, the impact of temperature on chloride ion diffusion in concrete at different ages still requires further investigation, especially in hot marine environments. Generally, the temperature in marine environments is above 20 ℃, with chloride ion concentrations of approximately 100 mol/m3. The chloride ion concentration in low-tide seawater is generally higher than that in high-tide seawater, and the chloride ion concentration in bottom seawater is generally higher than that in surface water. For example, the concentration of chloride ions in the Huangmao Sea area ranges from 92 mol/m3 to 349 mol/m3. The erosion of chloride ions in marine environments is the leading cause of durability issues in concrete. Concrete is subjected to physical and chemical erosion, reinforcement corrosion, concrete expansion, and spalling, leading to structural changes and severe deterioration in durability [17-21]. In recent years, the durability of concrete in marine environments has become a significant issue that needs to be addressed in the industry [22, 23].
This study aims to develop a mesoscale model to simulate chloride ion transport in saturated concrete, explicitly accounting for the distinct diffusion characteristics of its constituent phases. The research focuses on three key aspects: Investigating the effects of mineral admixtures and temperature on chloride ion diffusion in concrete; Analyzing chloride ion migration patterns by considering both the intrinsic material properties of concrete and temperature variations; Establishing quantitative relationships between these factors and chloride transport behavior to enhance durability predictions.
2. Experiment study
2.1. Materials and concrete mix proportion
The cement used is ordinary Portland cement with a grade of 42.5. The water-to-cement ratio is set at 0.4. The fine aggregate is river sand. The maximum particle size of the coarse aggregate is 26.5 mm, with continuous grading. The specific mix proportions of the concrete specimen are shown in Table 1.
Table 1Concrete mix proportion
NO. | Temperature (℃) | Material (kg/m3) | |||||
Cement | Slag | Fly ash | Sand | Gravel | Water | ||
ADM20 | 20 | 480 | 0 | 0 | 702 | 1248 | 192 |
ADM25 | 25 | 480 | 0 | 0 | 702 | 1248 | 192 |
ADM30 | 30 | 480 | 0 | 0 | 702 | 1248 | 192 |
BSF50 | 20 | 430 | 50 | 0 | 702 | 1248 | 192 |
BSF100 | 20 | 380 | 100 | 0 | 702 | 1248 | 192 |
BSF130 | 20 | 350 | 130 | 0 | 702 | 1248 | 192 |
FA40 | 20 | 440 | 0 | 40 | 702 | 1248 | 192 |
FA80 | 20 | 400 | 0 | 80 | 702 | 1248 | 192 |
FA120 | 20 | 360 | 0 | 120 | 702 | 1248 | 192 |
BSF130FA120 | 20 | 230 | 130 | 120 | 702 | 1248 | 192 |
2.2. Calculated methods
A three-dimensional spherical random aggregate model of concrete is created using the Monte Carlo method to obtain coarse aggregates that meet the grading requirements. These aggregates are randomly distributed within the concrete specimens, accounting for 60 % of the total volume. The Interfacial Transition Zone (ITZ) between the cement paste and the aggregate is designated to be 0.1 mm thick. Notably, the chloride ion diffusion coefficient within the ITZ is significantly higher compared to that of the cement mortar. In this paper, it is designed as three times greater than the diffusion coefficient in the cement mortar. Additionally, the diffusion coefficient for the aggregate is fixed at a value of 0. The activation energy was 11.92 kJ/mol. The aggregate information is imported into the COMSOL software to establish the geometric model of the concrete specimen, as shown in Fig. 1 and Fig. 2.
Fig. 1Randomly distributed aggregate
Fig. 2Mesh subdivision
The aggregate particle size distribution follows a randomized Fuller gradation, calculated using the following equation:
where is the aggregate size (mm); is the aggregate volume fraction (%), is the minimum particle size (mm), is the maximum particle size (mm), and is the normalization parameter. Using the generated aggregate size and the local coordinate system origin as reference, the spatial distribution of aggregate vertices can be stochastically determined through spherical coordinates as follows:
where is the Cartesian coordinate of the aggregate vertex, is spherical coordinates.
To clarify the influence of water-binder ratio, mineral admixtures and other factors on ion transport performance, this work takes chloride ion transport in saturated state as an example. According to the law of conservation of mass, the diffusion equation can be expressed as:
where represents the flow rate of chloride ion (mol·m-2·s-1), denotes the diffusion coefficient of chloride ion (m2·s-1), indicates the concentration of chloride ion (mol·m-3), and corresponds to the diffusion time (s). , and are the three reference directions of three-dimensional space.
The study focuses on analyzing the variation of chloride ion concentration along the depth of concrete under different influencing factors. In normal circumstances, physical model experiments use powder grinding to test the average chloride ion concentration of the same concrete at different depths. To provide more realistic results, COMSOL displays the concentration distribution of chloride ions in two-dimensional and three-dimensional contour plots. Graphing software can be used to obtain distribution of chloride ion concentration along the concrete depth at different ages.
2.3. Model validation
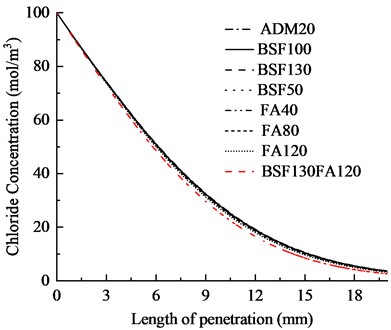
To verify the accuracy and reliability of the model proposed in this study, concrete specimens with aggregate volume fraction of 60 % were selected. The aggregate size was controlled in the range of 5-26.5 mm, and the concrete specimen size was 100 mm × 50 mm. The mix ratio is shown in Table 3. According to GBT50082-2024 “Standard Test Method for Long-term Performance and Durability of Concrete”, the content of free chloride ions at different depths of concrete under different erosion time was determined.
Table 2Concrete mix proportion and test results
Material (kg/m3) | Chloride ion diffusion coefficient (10-12m2/s) | |||||
Water-binder ratio | Cement | Slag | Fly ash | 20 ℃ | 25 ℃ | 30 ℃ |
0.38 | 155 | 90 | 120 | 1.93 | 2.25 | 2.7 |
0.36 | 280 | 140 | 0 | 1.34 | 1.63 | 1.84 |
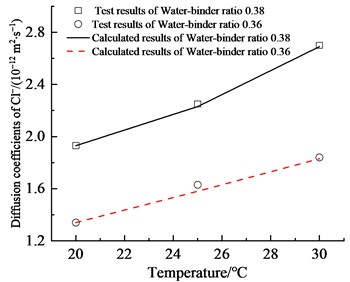
In this study, a concrete model with the same test parameters was established. The chloride diffusion coefficients obtained from the model calculations were compared with those measured during experiments, as illustrated in Fig. 3. It was observed that as the temperature increased, both the apparent chloride ion diffusion coefficient calculated by the model and that measured through tests exhibited a gradual rise. A comparison of the simulation results with the experimental data reveals a strong correlation, with the overall error remaining below 5 %. This indicates that the model developed in this study can effectively predict the transport behavior of chloride ions in concrete. To further investigate the influence of temperature and mix ratios on the chloride ion transport properties in concrete at the meso-scale, this work will use this model to carry out parametric studies on the characteristics of different temperatures and mineral admixture contents.
Fig. 3Comparison between calculated results and test results
3. Results and discussion
3.1. Effect of age on concrete chloride diffusion
The internal chloride ion concentration in reinforced concrete can be influenced to some extent by the duration of the external chloride ion attack. As time progresses, the concentration of chloride ions within the cement mortar increases. The longer the time, the more pathways are available for chloride ions to transport and the weaker the resistance to chloride ion movement within the concrete. For analysis, the chloride ion concentration is set at 100 mol/m3, and the critical concentration of chloride ions is 0.13 %. The termination time is 28 days, with a time step of 1 day. Changing the time factor analyses the migration of chloride ions under different time conditions.
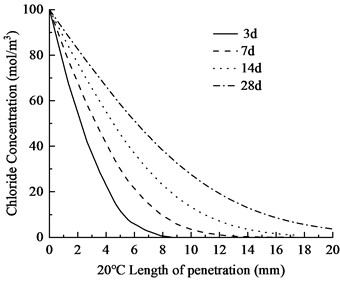
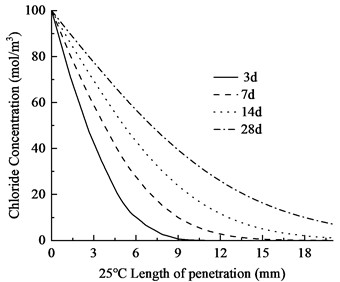
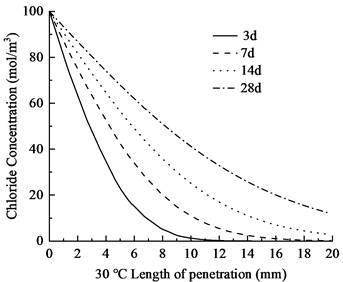
Fig. 4Chloride ion distribution in concrete at varying temperatures
a) ADM20
b) ADM25
c) ADM30
The temperature is set at 20 ℃, 25 ℃, and 30 ℃, respectively, and the chloride ion concentration maps and contour plots at 3, 7, 14, and 28 days are generated. The distribution of chloride ion concentration along the depth of eroded concrete is shown in Fig. 3. The chloride ion penetration depths in the concrete are provided in Table 4.
Table 3Chloride penetration depth (mm)
No. | 3 d | 7 d | 14 d | 28 d |
ADM20 | 3.01 | 4.39 | 6.05 | 8.20 |
ADM25 | 3.34 | 4.98 | 6.88 | 9.62 |
ADM30 | 3.85 | 5.64 | 7.85 | 11.06 |
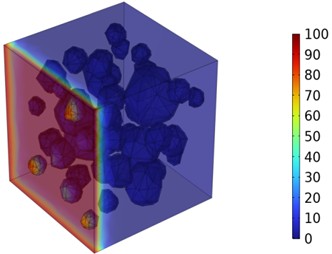
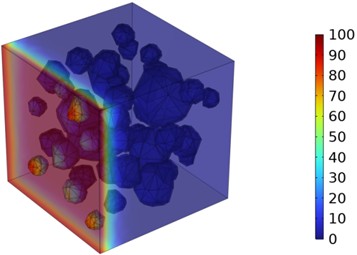
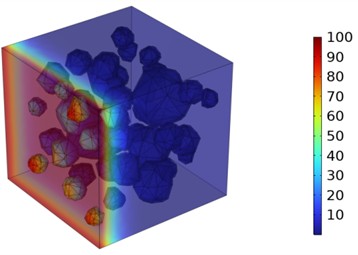
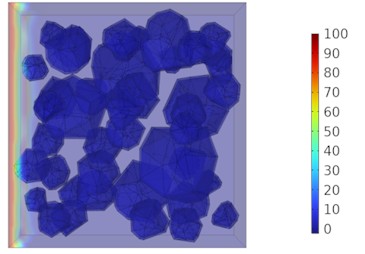
As shown in Fig. 4 and Table 3, the chloride ion penetration depth in concrete increases by 10.9 % at 25 °C and 27.9 % at 30 °C compared to 20 °C at 3 days. This increase continues to 13.4 % (25 °C) and 28.5 % (30 °C) after 7 days, further rising to 13.7 % (25 °C) and 29.8 % (30 °C) after 14 days. By 28 days, the penetration depth increases by 17.3 % at 25 °C and 34.9 % at 30 °C. Notably, at 30 °C, the chloride penetration reaches 11.06 mm. The 28-day chloride penetration depth reaches 8.20 mm at 20 °C, with chloride concentration remaining below 5 mol/m3 at 20 mm depth. This represents a 2.86 mm increase in penetration depth and a 15 mol/m3 concentration difference at 20 mm depth compared to earlier measurements. The data clearly demonstrate that elevated temperatures accelerate both chloride penetration depth and erosion rate in concrete. Notably, temperature exerts a substantially greater influence on concrete durability.
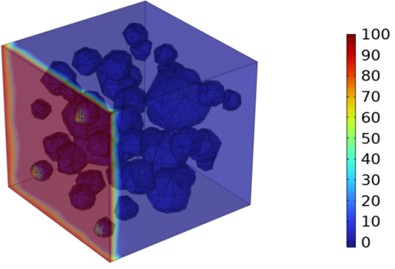
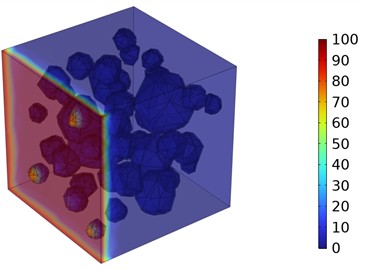
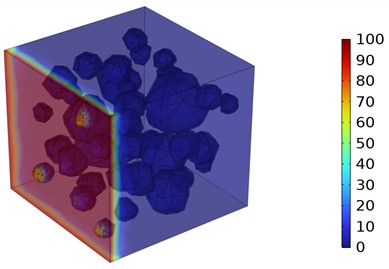
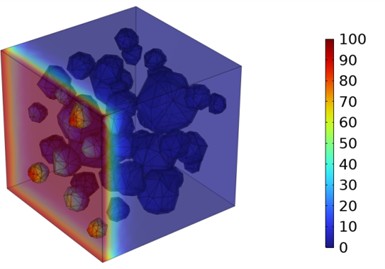
Fig. 5Three-dimensional cloud maps of chloride ion concentration distribution (20 ℃)
a) 3 d
b) 7 d
c) 14 d
d) 28 d
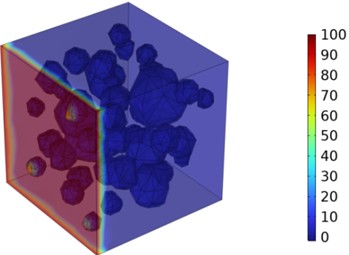
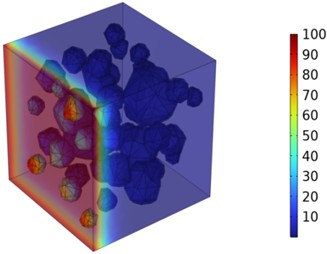
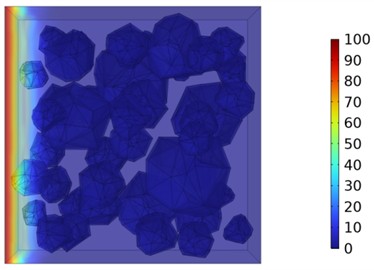
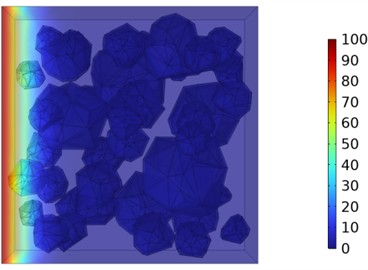
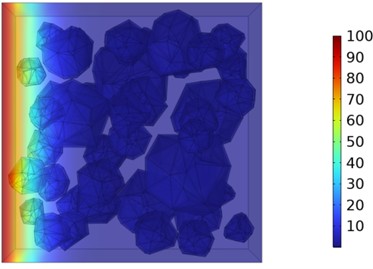
Figs. 5-7 illustrate the three-dimensional distribution of chloride ion concentration in concrete at 20 °C, 25 °C, and 30 °C, respectively, while Figs. 8-10 present the corresponding two-dimensional distributions. The chloride ion erosion process at 20°C is simulated in Figs. 5(a-d) and 8(a-d) for curing periods of 3, 7, 14, and 28 days. With increasing concrete age, the expansion of red regions in these figures demonstrates progressive chloride ion penetration. Notably, the temperature effect becomes more evident at higher temperatures (25 °C and 30 °C) in Figs. 6-7 compared to Fig. 5 (20 °C), clearly visualizing how elevated temperatures accelerate chloride ion erosion in concrete.
Fig. 6Three-dimensional cloud maps of chloride ion concentration distribution (25 ℃)
a) 3 d
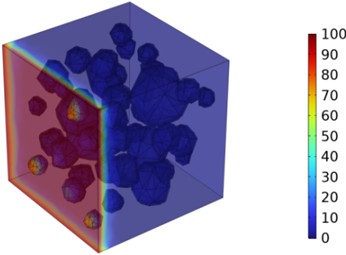
b) 7 d
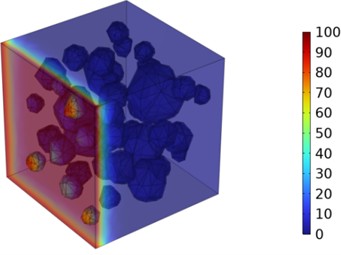
c) 14 d
d) 28 d
Fig. 7Three-dimensional cloud maps of chloride ion concentration distribution (30 ℃)
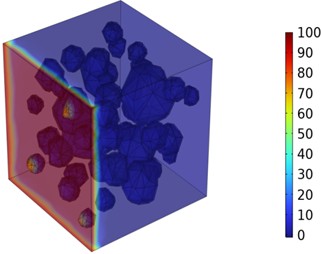
a) 3 d
b) 7 d
c) 14 d
d) 28 d
Fig. 8Two-dimensional cloud maps of chloride ion concentration distribution (20 ℃)
a) 3 d
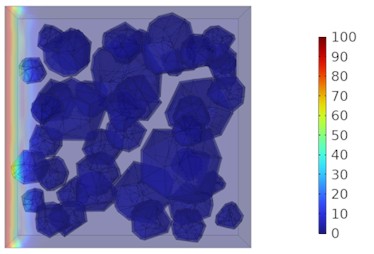
b) 7 d
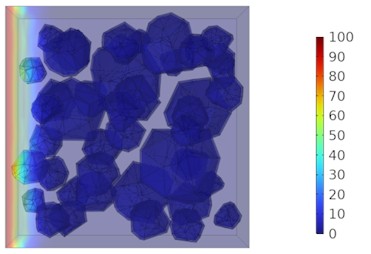
c) 14 d
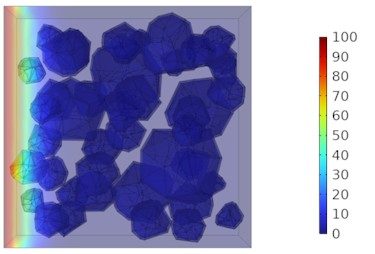
d) 28 d
Fig. 9Two-dimensional cloud maps of chloride ion concentration distribution (25 ℃)
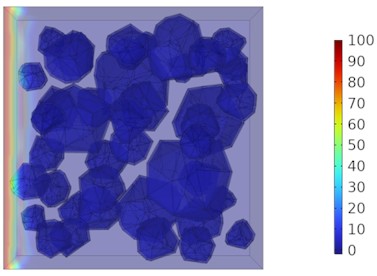
a) 3 d
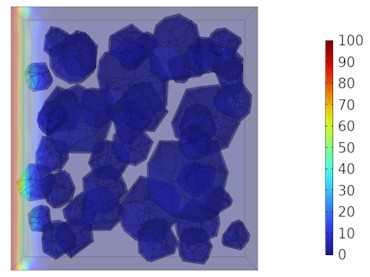
b) 7 d
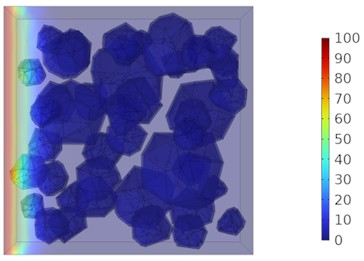
c) 14 d
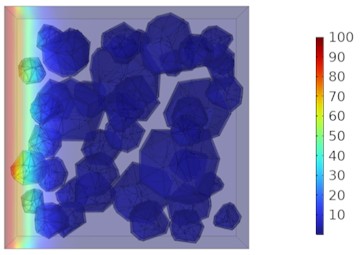
d) 28 d
Fig. 10Two-dimensional cloud maps of chloride ion concentration distribution (30 ℃)
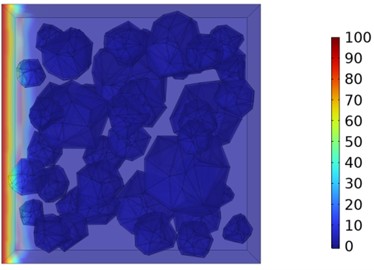
a) 3 d
b) 7 d
c) 14 d
d) 28 d
3.2. Effect of temperature on chloride ion transport rate in concrete
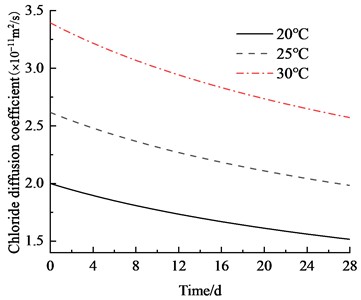
Fig. 11 illustrates the chloride ion concentration and its distribution along the depth at temperatures of 20 ℃, 25 ℃, and 30 ℃. The initial diffusion coefficient of chloride ions in concrete changes with temperature variations: it is 2×10–11 m2/s at 20 ℃; it increases to 2.62×10–11 m2/s at 25 ℃; and it further rises to 3.41×10–11 m2/s at 30 ℃. Compared with the results at 20 ℃, which increase by 31 % and 70.5 % at 25 ℃ and 30 ℃, respectively, Fig. 10 indicates a positive correlation between the initial diffusion coefficient and temperature.
Fig. 11Chloride ion diffusion coefficient of water binder ratio of 0.4
3.3. Impact of mineral admixtures on chloride ion transport in concrete
Ground granulated blast furnace slag shares a chemical composition similar to ordinary Portland cement. When incorporated into cement, its reactivity is fully utilized, optimizing the internal pore structure and significantly enhancing the concrete’s density and impermeability.
Fig. 12Effect of different mineral powder content on chloride ion penetration depth of concrete
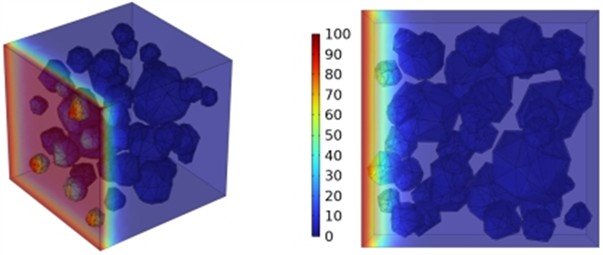
a) ADM20
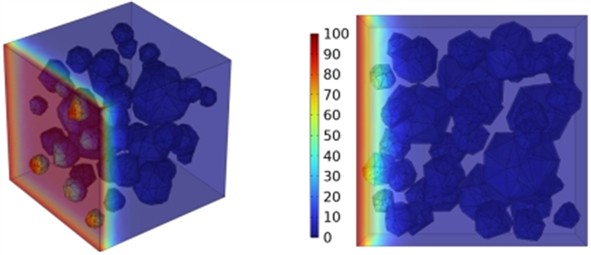
b) BSF50
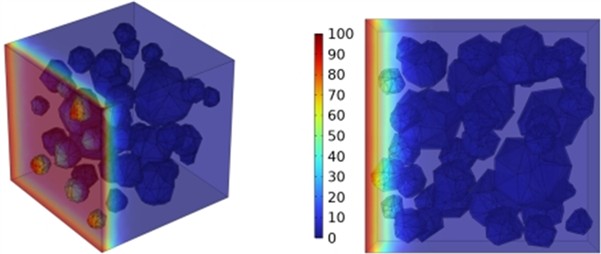
c) BSF100
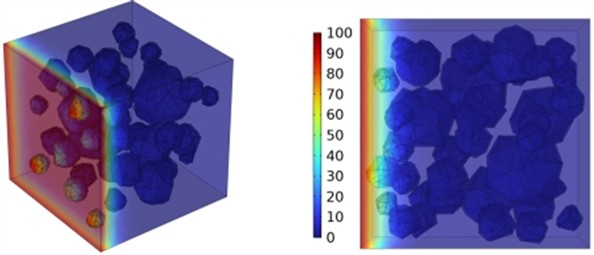
d) BSF130
The meso-scale results demonstrate that with slag contents of 10.4 %, 20.8 %, and 27.1 %, the chloride ion diffusion coefficient of concrete exhibits reductions of 0.2 %, 0.4 %, and 0.6 %, respectively at 3 days; 0.5 %, 0.9 %, and 1.3 % at 7 days; 0.8 %, 1.6 %, and 2.4 % at 14 days; and 1.4 %, 2.7 %, and 4.1 % at 28 days, respectively. The results at 3, 7, and 14 days indicate that the GGBFS content has a limited impact on concrete's early-stage chloride ion resistance. Fig. 12 displays the cloud map of chloride ion concentration distribution in concrete with varying slag contents at 28 days. Beyond 28 days, the effect of slag becomes more pronounced as the curing age increases. The resistance to chloride ion penetration improves progressively with higher slag content, as illustrated in Fig. 12.
3.4. Impact of fly ash on chloride ion migration in concrete
The chloride ion diffusion coefficients of concrete decreased with increasing fly ash content (8.3 %, 16.7 %, and 25 %), showing reductions of 0.3 %, 0.8 %, and 1.4 % at 3 days; 0.7 %, 1.8 %, and 3.1 % at 7 days; 1.2 %, 3.2 %, and 5.5 % at 14 days; and 2.1 %, 5.4 %, and 9.2 % at 28 days, respectively. These results suggest that fly ash has a relatively minor effect on chloride ion resistance during the early stages (3-14 days).
Fig. 13Effect of varying fly ash content on chloride ion penetration depth in concrete
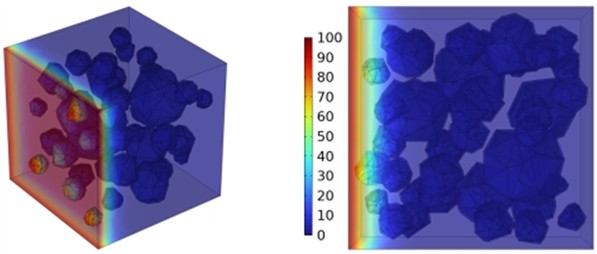
a) FA40
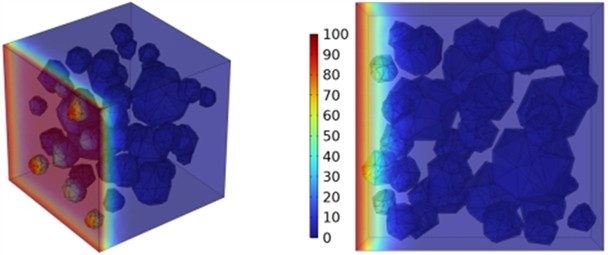
b) FA80
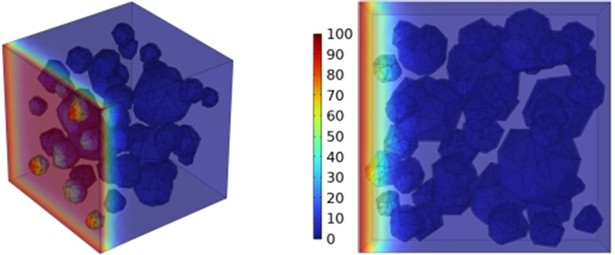
c) FA120
As illustrated in Fig. 13, higher fly ash proportions led to a gradual reduction in chloride ion penetration depth at 28 days. This improvement is primarily due to the presence of glassy microspheres in fly ash, which decrease water demand, enhance workability, and refine the concrete’s pore structure and density. Furthermore, fly ash demonstrates superior effectiveness in improving chloride ion resistance compared to slag.
3.5. Impact of combined slag and fly ash on chloride ion transport in concrete
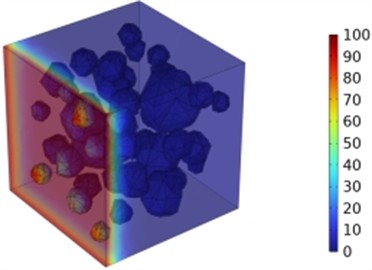
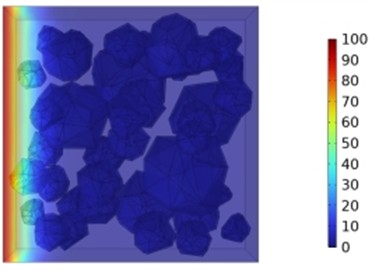
The incorporation of either slag (10.4 %, 20.8 %, 27.1 %) or fly ash (8.3 %, 16.7 %, 25 %) individually improved the chloride ion resistance of concrete. However, the combined use of 27.1 % slag and 25 % fly ash yielded the most substantial enhancement, reducing chloride ion diffusion coefficients by 3.1 %, 7.1 %, 12.4 %, and 20.2 % at 3, 7, 14, and 28 days, respectively. This synergistic effect also led to a significant decrease in chloride ion penetration depth, as demonstrated in Figs. 14 and 15. Further analysis revealed that a water-to-binder ratio of 0.4, when combined with 27.1 % slag and 25 % fly ash, optimally enhanced the concrete's resistance to chloride ion penetration.
Fig. 14Impact of mineral admixtures on concrete erosion resistance
a) Chloride ion concentration distribution
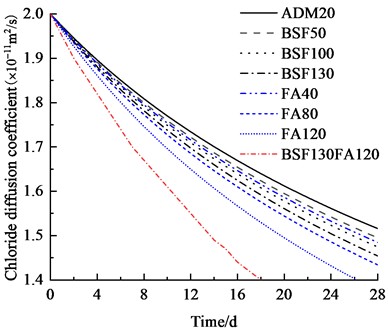
b) Chloride ion diffusion coefficient
Fig. 15Effect of combined fly ash and slag on chloride ion penetration depth in concrete
4. Conclusions
This study utilizes the Monte Carlo method to develop a stochastic aggregate model of concrete, enabling numerical simulation of chloride ion penetration behavior. The key findings are summarized as follows:
1) The mesoscale finite element analysis model, constructed through Monte Carlo simulation, successfully characterizes chloride ion transport in concrete under varying temperature conditions while evaluating the effectiveness of mineral admixtures in improving chloride resistance.
2) Experimental results demonstrate an age-dependent reduction in the chloride diffusion coefficient, indicating progressive enhancement of concrete's chloride resistance over time.
3) Binary mineral admixture systems exhibit superior performance compared to single-admixture formulations. Both slag and fly ash effectively reduce chloride diffusivity, with comparative analysis revealing fly ash’s greater efficacy in enhancing chloride resistance relative to slag.
4) Optimization studies identify the composite admixture of 27.1 % slag and 25 % fly ash as the most effective formulation for minimizing chloride diffusion coefficients and maximizing resistance. These findings demonstrate the synergistic potential of combined mineral admixtures in improving concrete durability against chloride-induced deterioration.
References
-
J.-J. Chen, R.-J. Wu, K. Chen, Z. Wang, and J. Xia, “Influence of initial saturation degree on chloride transport in concrete under hydraulic pressure,” Journal of Building Engineering, Vol. 87, No. 25, p. 108897, Jun. 2024, https://doi.org/10.1016/j.jobe.2024.108897
-
L. Wu, C. Yi, Q. Feng, X. Huang, and Z. Mao, “Numerical simulation of sulfate attack in cement based materials: Considering dynamic boundary calcium concentration,” Case Studies in Construction Materials, Vol. 21, No. 18, p. e04091, Dec. 2024, https://doi.org/10.1016/j.cscm.2024.e04091
-
C. Jiang, L. Jiang, X. Tang, J. Gong, and H. Chu, “Impact of calcium leaching on mechanical and physical behaviors of high belite cement pastes,” Construction and Building Materials, Vol. 286, p. 122983, Jun. 2021, https://doi.org/10.1016/j.conbuildmat.2021.122983
-
L. Liu, S. Zhao, J. Xin, and Z. Wang, “Simplified analysis of thermal cracks in low‐heat portland cement concrete,” Advances in Civil Engineering, Vol. 2022, No. 1, p. 76305, Apr. 2022, https://doi.org/10.1155/2022/7630568
-
O. H. A. Dehwah and Y. Xi, “Theoretical model for the coupling effect of moisture transport on chloride penetration in concrete,” Cement and Concrete Research, Vol. 177, p. 107431, Mar. 2024, https://doi.org/10.1016/j.cemconres.2024.107431
-
B. Bary, A. Machner, and K. de Weerdt, “Simulation of chloride diffusion tests in cement paste and mortar made with CEM II and CEM VI cements,” Cement and Concrete Research, Vol. 183, p. 107559, Sep. 2024, https://doi.org/10.1016/j.cemconres.2024.107559
-
R. Cherif, A. E. A. Hamami, A. Aït-Mokhtar, and W. Bosschaerts, “Thermodynamic equilibria-based modelling of reactive chloride transport in blended cementitious materials,” Cement and Concrete Research, Vol. 156, p. 106770, Jun. 2022, https://doi.org/10.1016/j.cemconres.2022.106770
-
Z. Zhuang, S. Mu, Z. Guo, G. Liu, J. Zhang, and C. Miao, “Diffusion-reaction models for concrete exposed to chloride-sulfate attack based on porosity and water saturation,” Cement and Concrete Composites, Vol. 146, p. 105378, Feb. 2024, https://doi.org/10.1016/j.cemconcomp.2023.105378
-
J. Xia, K. Chen, S. Hu, J. Chen, R. Wu, and W. Jin, “Experimental and numerical study on the microstructure and chloride ion transport behavior of concrete-to-concrete interface,” Construction and Building Materials, Vol. 367, p. 130317, Feb. 2023, https://doi.org/10.1016/j.conbuildmat.2023.130317
-
Y. Hu, S. Hu, Y. Ye, W. Li, and C. Li, “Time-varying analytical model and mesoscopic numerical simulation of chloride ion diffusion in prestressed concrete cylinder pipe,” Journal of Building Engineering, Vol. 94, p. 109951, Oct. 2024, https://doi.org/10.1016/j.jobe.2024.109951
-
X. Du, L. Jin, and G. Ma, “A meso-scale numerical method for the simulation of chloride diffusivity in concrete,” Finite Elements in Analysis and Design, Vol. 85, pp. 87–100, Aug. 2014, https://doi.org/10.1016/j.finel.2014.03.002
-
B. Li, J. Mao, T. Nawa, and Z. Liu, “Mesoscopic chloride ion diffusion model of marine concrete subjected to freeze-thaw cycles,” Construction and Building Materials, Vol. 125, pp. 337–351, Oct. 2016, https://doi.org/10.1016/j.conbuildmat.2016.08.052
-
H. Cui, L. Cao, X. Cao, S. Yu, and M. Huang, “Experimental investigation of chloride ion monitoring in concrete considering the coupling effect of temperature and humidity,” Journal of Building Engineering, Vol. 98, p. 111155, Dec. 2024, https://doi.org/10.1016/j.jobe.2024.111155
-
G. H. Guo, Y. F. Bai, and T. Wang, “Analysis of dynamic load level of high-speed heavy vehicle imposed on uneven pavement,” in Applied Mechanics and Materials, Vol. 138-139, pp. 146–152, Nov. 2011, https://doi.org/10.4028/www.scientific.net/amm.138-139.146
-
T. Du, J. Xiao, C. Li, Y. Gan, and X. Jiang, “Experimental and numerical study on the chloride ions penetration in recycled aggregate concrete,” Construction and Building Materials, Vol. 451, p. 138702, Nov. 2024, https://doi.org/10.1016/j.conbuildmat.2024.138702
-
J. Ying, W. Chen, S. Chen, and B. Chen, “Chloride ion diffusion in recycled concrete containing slag under biaxial compression,” Construction and Building Materials, Vol. 454, p. 139136, Dec. 2024, https://doi.org/10.1016/j.conbuildmat.2024.139136
-
S. Chen, “Study on chloride ion transport model of hydraulic concrete modified by multiple factors,” Water Conservancy Science and Technology and Economy, Vol. 30, No. 11, pp. 127–130, Nov. 2024, https://doi.org/10.3969/j.issn.1006-7175.2024.11.025
-
X. Jiang, “Performance Analysis of Concrete for Bridge in Cold Area,” Communications Science and Technology Heilongjiang, Vol. 47, No. 11, pp. 111–114, 2024, https://doi.org/10.16402/j.cnki.issn1008-3383.2024.11.033
-
L. Jin, J. Yang, J. Wu, and X. Du, “Probabilistic prediction model of fatigue life of RC structures considering the meso-scale inhomogeneity of concrete,” Materials Reports, Vol. 38, No. 20, pp. 161–168, Oct. 2024, https://doi.org/10.11896/cldb.23090009
-
A. Weng, “Study on diffusion model of water molecules and chlorideions in fly ash-based geopolymer and its application,” Anhui University of Science and Technology, 2024.
-
W. Zhang, G. Sheng, and L. Wang, “A review of the property evolution of cement-based materials for ballastless track under complex service environment,” Materials Reports, Vol. 38, No. 22, pp. 143–160, 2024, https://doi.org/10.11896/cldb.23080140
-
J.-J. Chen, Q.-F. Liu, W.-L. Jin, and J. Xia, “Experiment and simulation on the coupled effects of calcium leaching and chloride transport in concrete under hydraulic pressure,” Cement and Concrete Composites, Vol. 155, p. 105834, Jan. 2025, https://doi.org/10.1016/j.cemconcomp.2024.105834
-
Y. Wei, Z. Chen, M. Yio, C. Cheeseman, H. Wang, and C. S. Poon, “Advanced moisture control in porous aggregates for improved lightweight high-performance concrete,” Cement and Concrete Composites, Vol. 155, p. 105826, Jan. 2025, https://doi.org/10.1016/j.cemconcomp.2024.105826
About this article
This research was funded by the Basic Research Business Fee Project of Central Public Welfare Research Institutes (2024-9048). The authors also wish to express their acknowledgement to the research fund of Road& Bridge South China Engineering Co., Ltd. for their financial support.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Dongchang Wen: conceptualization, formal analysis, investigation, methodology, visualization, writing-original draft preparation. Guohe Guo: writing-original draft preparation, methodology, investigation. Shangchuan Zhao: writing-original draft preparation, methodology, investigation. Longlong Liu: writing-original draft preparation, investigation.
The authors declare that they have no conflict of interest.
